Quality, an essential component of production monitoring, is a transverse function that operates from the receipt of raw materials to the delivery of products.
For the manager
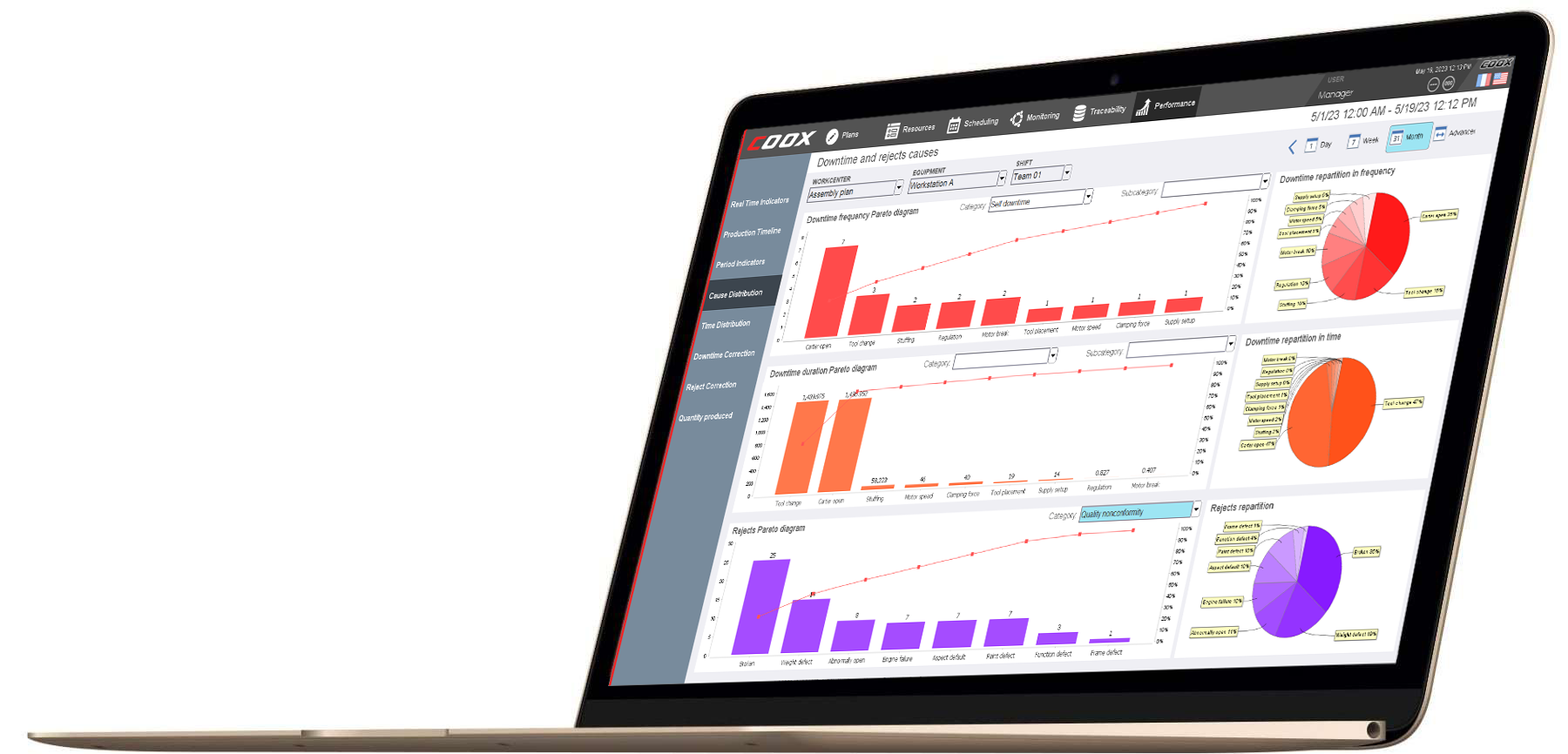
Quality monitoring no longer requires constant verification of operator information and time-consuming maintenance of spreadsheets for compiling statistics. COOX (COllaborative Operation & eXecution) performs all these operations automatically and associates them automatically with the production batch files. You can also create new control ranges without using programming. The SPC / SQC module allows you to push even further and prevent non-qualities.
For the operator
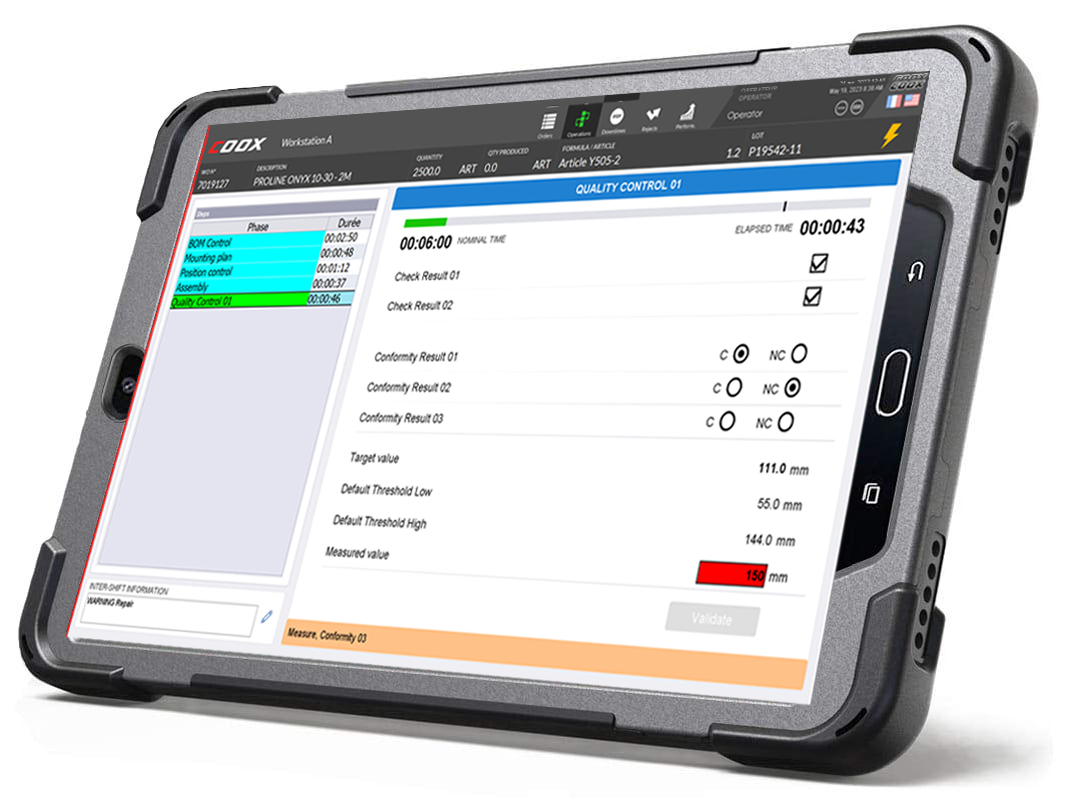
No more paper entry of compliance sheets: the risk of error is reduced to a minimum as well as the time for entering information via an intuitive interface. Information is collected directly on the equipment whenever possible: weighing, size, etc. Sampling is facilitated for the implementation of SPC / SQC controls.
Quality controls on receipt
On receipt, one or more checks are carried out to know which batch of raw materials or components is accepted or rejected. These controls can be manual, automatic (the data are acquired directly from the equipment) or hybrid (the operator will launch and / or record control operations carried out by an autonomous equipment. The parameters of these controls are traced.
Quality controls online or offline
Depending on the processes and constraints (environmental, legislative, customer specifications) that affect the products, quality controls must be carried out in a systematic manner (online) or by sampling (offline). The sampling control uses statistical methods (SQC : Statistic Quality Control) analogous to statistical process control (SPC) methods. Different types of control cards can be implemented, as well as controls with several tolerance levels (eg TU1 / TU2). Production quality controls can be manual, automatic or hybrid. Interfaces with the operator are customizable.
Quality rate and performance
In terms of performance, production losses induced by non-quality play a crucial role. The quality rate is one of three componenets of OEE (Overall Equipment Effectiveness) that measures the productivity of a line or a production unit. It is calculated automatically according to the measurement (automatically and / or by input) of the products rejected at different steps of manufacture. Thanks to a real-time connected and controlled tool, the operator is immediately notified of a malfunction and is able to implement the necessary corrections. He is thus at the heart of continuous improvement : the tool helps him achieve his objectives (produce for example at the right pace), without compromising on the quality of the products.

CORRESPONDING MODULES OF THE COOX® RANGE
Quality monitoring is based on MESbox PMT module (Production Management & Traceability) and MESbox QPI (Quality Performance & Indicators) of the COOX range.
• Monitoring quality, quality rates, and discards
• Calculating standard OEE and related indicators, or custom performance indicators
• Calculating standard MTBF (mean time between failures), MTTR (mean time to repair), or custom maintenance indicators
• Monitoring materials consumption and assessments
• Automatic acquisition or manual entry of information
• Support for SQL Server Oracle™ and MySQL™ databases
• Output formats: PDF™, Excel™ and texte
• Generation and distribution of dynamic graphs formulated with Excel™
• Predefined models and creation of report models
• Customizable report options established in operations (filters, selections, etc.)

Process and operating modes monitoring is provided by MESbox PMT module (Production Management & Traceability) of the COOX range.
• Runtime software for batch and discreet processes
• Conformity ISA-88/ISA-95
• Graphic modeling of the process without data-processing knowledge
• Ergonomics of control customizable
• Detailed planning of the work orders
• Traceability of the set points and actual value, batch file
• Automatic quantitative adjustment
• Visualization and piloting since any point of the intranet
• Direct link with the traceability of product
• FDA requirements 21 CFR 11


